By Dave Harrold
Do you know what you get when you mix S88, S95, B2MML, M2Pack, BatchML, MES, OPC, JETT, OMAC and PAT together? No, it’s not alphabet soup. What you get is a collection of tools that, when used in combination, provide proven, well-documented ways to create a flexible, robust production environment that fulfills manufacturers’ long-time desire to obtain near real-time production visibility.
Greyssi Campos, a control systems engineer with Wyeth-Ayerst Lederle in Carolina, Puerto Rico, knows firsthand what S88 can provide. “Before we implemented S88, each recipe change required new code, new graphics and validation of both, in addition to validation of the recipe,” says Campos. “S88 has saved us a tremendous amount of time and money by eliminating code and graphic validation when recipes are changed.”
An Internet search for S88, S95, B2MML, M2Pack, BatchML, MES, OPC, JETT, OMAC and PAT returns thousands of hits for books, articles, whitepapers and tutorials describing these standards, templates and initiatives, but you’ll find very little on why using them makes savvy business sense—until now.
In his book, Management Challenges for the 21st Century, business guru Peter Drucker explains it’s best to focus on the meaning of the information and not on the technology that collects and delivers it. Drucker’s sage advice is exactly why using this collection of standards, templates and initiatives can help:
- Increase process knowledge,
- Improve quality, yield and throughput,
- Reduce production costs,
- Reduce equipment lifecycle hosts.
S88/S95 Integrated
Functional Hierarchy
While this structure represents production, S95 supports its easily being adapted and applied to maintenance, quality, or inventory information
Meet the Pieces
Certainly an Internet search on these individual tools will provide significantly more detail about each. However for our purposes, the following simple definitions are adequate to understand why using them together can improve the visibility of executives into production processes.
Standards and templates. S88 defines the terminology as well as the physical and functional hierarchy for recipe management and process segmentation. Use of S88 encourages development and reuse of pretested software modules designed to match the capabilities of equipment and processing units. Originally developed for batch processes, S88 has proven to be an equally effective automation development model for continuous processes.
S95 consists of terminology and models that can be used to develop an efficient information exchange between a variety of business systems, such as sales, finance, quality, production, maintenance and logistics. S95 encourages use of the standard business system information exchange language, or unified modelling language (UML).
Manufacturing execution systems (MES) are defined in S95 as systems that control “level 3-” related activities, such as resource allocation, production dispatching, collection of product production activities, quality assurance management, document management, labor management and more.
Communications. B2MML is a set of XML schemas developed by a World Batch Forum working group. Using the eXtensible Markup Language (XML) schema definition language, B2MML schemas are available as royalty-free, ready-to-use templates designed to make implementation of the S95 standard easy.
Batch markup language (BatchML) provides explanatory information about developing XML schemas that may be used to exchange information about recipes, equipment and batch lists per the data models and attributes defined in the S88 standard.
OPC is an open communication protocol for industrial automation and the enterprise systems that support industry. Interoperability is assured through creation and maintenance of open standards specifications. Based on fundamental standards and technology of the general computing market, OPC Foundation adapts and creates specifications that fill industry-specific needs.
Initiatives Underway. The Joint Equipment Transition Team (JETT) is a consortium of healthcare industry manufacturers, equipment suppliers and consultants seeking to improve communications between users and suppliers to more effectively meet the validation requirements of the healthcare industries as defined in version 4.0 of the ISPE’s Good Automated Manufacturing Practice Guide.
Make2Pack is a joint effort by several industry groups to harmonize similar standards and produce a common framework of terminology and models that supports automation strategies across the entire manufacturing landscape—batch and continuous processing, fill-finish and packaging.
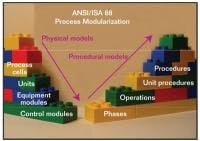
ANSI/ISA S88 Process Modueles
S88 enables reuse of pretested software modules that match capabilities of devices and processing units.
Manufacturing Enterprise Solutions Association (MESA) International is an organization of manufacturers and information system providers focused on leveraging the application layer defined in S95 as level 3.
Open, Modular Architecture Control (OMAC) Users Group was established in 1994 to produce consensus guidelines to improve flexibility, improve capability and reduce system integration costs in the development and implementation of open control technologies for manufacturing applications.
Process and Analytical Technology Initiative (PAT) is an effort by the U.S. Food and Drug Administration that encourages development and use of systems for analyzing and controlling manufacturing processes based on timely measurements of critical quality and performance attributes obtained during the processing of raw and in-process materials with the goal of ensuring end product quality.
Okay, now that we have some idea of the pieces, let’s examine how they fit together to improve plant-floor visibility; but first a word to the naysayers.
Excuses, Excuses
Too frequently people believe that their businesses and/or processes are unique and, therefore, not adaptable to standardized information integration solutions. They should heed Drucker’s advice to focus on the meaning of information, not the delivery mechanism. To illustrate his point, Figure 1 provides a graphical representation of what an integrated S88/S95 and B2MML schema solution provides. If you can see your company’s production-to-business-systems architecture fitting into this hierarchy, then the standards, templates and initiatives we’re discussing can:
- Increase your company’s process knowledge,
- Improve process and product quality, yield and throughput,
- Reduce production costs,
- Reduce equipment life-cycle costs.
If you’re still reading, chances are you believe S95 and B2MML could improve visibility into the bowels of your business. However, despite Figure 1’s insight into what S95 and B2MML bring to the solution, it does nothing to answer the question—why S88?
Why S88?
Applying the principles described in S88 becomes most beneficial if:
- You’re using the same equipment to produce entirely different products at different times,
- You need to track batch lots,
- Your customers require documented proof that your products meet pre-defined specifications,
- You need the ability to transfer product production to different facilities,
- You need the flexibility of assigning control system application support personnel (or contractors) to multiple systems, processes and sites.
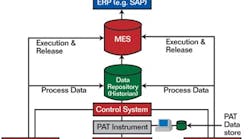
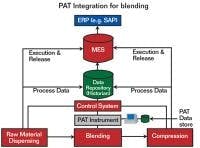
Where fits into the system
A successful PAT implementation combines data from many sources in the control layer.
Courtesy of Rockwell Automation.
An examination of control system software developed pre-S88 usually reveals “spaghetti code.” Within spaghetti code, it’s often possible to see remnants of a rudimentary structure that either wasn’t well-understood by subsequent software developers and/or simply wasn’t robust enough to accommodate years of process-control changes by multiple users. In such situations, the physical control system frequently gets the blame when, in fact, the real culprit is how the control system’s application software was implemented to begin with.
S88, illustrated in Figure 2, is a independent consensus control system standard that defines in great detail a common terminology, as well as the physical and procedural hierarchy for recipe management and process segmentation. The application of S88 models encourages development and reuse of pretested software modules designed to match the capabilities of equipment and processing units.
Originally developed by a committee of process control experts working toward a universal batch process solution, S88 has since proven an equally effective automation development model for continuous (e.g., refineries), as well as discrete processes (e.g., fill-finish).
The wide-spread acceptance of S88 is revealed by an examination of the underpinnings of every major control system on the market today. Though each vendor has incorporated its own unique representation of the S88 models into its control system offerings, the differences are mostly cosmetic. This nearly universal implementation of S88 models across different manufacturers’ control system platforms provides end users with significant advantages.
- Control system engineers with a good understanding of S88 can efficiently apply their application expertise across different manufacturers control systems.
- Application solutions are designed to maximize the features and benefits of processing equipment, even if not all features and benefits are required for every product produced.
- Modules successfully developed, validated and applied at one site are easily shared with other sites, often with little or no re-validation efforts necessary.
- In-process production status is shared more easily and consistently with MES and other business systems.
This last bullet is the focus of this article.
Because S95 is designed to be S88-aware, the application of S88 terminology and models in developing the control system application software makes it much easier to provide business systems with information whose origin is, in S95 terms, Level 0,1,2 plant-floor and/or Level 3 MES systems. That’s not to say the benefits of S95 can’t be obtained without having implemented an S88-conformant application, but it does mean you probably won’t be able to take full, if any, advantage of pre-configured, pre-tested templates such as B2MML. It also means you’ll need to be much more diligent in maintaining and validating your custom implementation over time.
The question facing some users is, “Do I fund and staff a one-time effort to redo my control system applications to conform to S88?” or “Do I develop a custom S95 implementation that requires an unpredictable amount of resources to maintain and update?”
Of course, there’s no single right answer, but at least part of the best decision for you lies in how you answer the following question, “What would be the impact on my current control system application software if my company decided to begin producing a completely different product using the existing equipment and piping in this facility?”
If the facility was S88-conformant, the changes would occur at the recipe level, and the existing instrumentation and control system validation qualifications would remain unchanged. That sounds simple, but the impact is huge, especially when measured in time to market.
Down PAT
One more set of letters in this alphabet soup remains to be discussed—PAT. Though the PAT initiative is officially targeted at FDA-regulated processes, its goal is universal throughout the process industries—to enhance the understanding and control of the manufacturing process in such a way that product quality is designed in.
When it comes to compliance to U.S. FDA regulations, cost is a major driver for companies in the life-sciences industry.
Prior to the FDA’s August 2002 announcement of Pharmaceutical Current Good Manufacturing Practices (cGMPs) for the 21st Century, companies conducted quality testing of veterinary and human drugs and select human biological products after the products were produced. This post-production quality testing practice adds cost in the areas of product scrap and rework, “in-process” inventory management, and delays in shipping finished product.
PAT, a key element in the FDA’s announcement, aims to increase the likelihood of defect-free pharmaceuticals by applying a risk-based approach that combines in-situ and other in-process quality measurement technologies alongside best practices that are designed to identify out-of-spec conditions as early in the production cycle as possible.
Referring to Figure 1, we see that MES software provides a solid framework for achieving the FDA’s goals for PAT. Though MES software is not likely to provide the entire PAT solution, it does form the foundation for assembling the results of other quality measurement tools, such as Six Sigma, SPC, lean manufacturing and kanban.
Certainly some users are already using such tools, but they may not be integrated and optimized to give maximum real-time visibility of plant-floor information to the enterprise.
A successful PAT implementation requires volumes of data, often from disparate systems. Such a data exchange environment is exactly what S95 and B2MML are designed to provide.
Proofs
All this sounds well and good, but you’ve heard such claims before. What is usually helpful is knowing how other companies are achieving success using integrated S88/S95 with B2MML solutions.
In 2003, Caracas, Venezuela-based food-and-beverage giant Empresas Polar reported it had successfully connected, in a few weeks time, its SAP PP-PI application to the plant floor using the S95 standard and B2MML schemas. At that time, project manager Francisco Ferrero said, “Our previous success using S88 provided us significant confidence to apply S95 and B2MML. Its clear, flexible, logical structure eliminated time-consuming discussions and allowed us to get to work.”
Arne Svendsen of Danish food giant Arla Foods says, “We view B2MML as the ‘wallbreaker’ for integrating complex, proprietary applications.”
Sandra Vann, of the Dow Co. in Midland, Mich., is a proponent of using S95 modelling to gather information that not only makes Six Sigma analysis better, but also reveals the right areas for additional study and improvements.
In accepting ISPE’s “Facility of the Year Award in Project Execution,” Oceanside, Calif.-based Genentech credited S88 and S95 as key elements that lead to its successful project.
Following a thorough investigation at its Oak Point operation in Belle Chasse, La., Chevron Chemical Co. is using production and batch management software and the S88 methodology to implement an MES to manage the full life cycle of production work orders, including manual, semi-automatic and automatic processes.
A very successful Make2Pack demonstration was hosted in 2006 by Proctor & Gamble in Cincinnati, Ohio. Following that demonstration, P&G representatives Dave Chappell, Rob Aleksa and Skip Holmes explained that P&G had been joined by SAB Miller, Unilever, Hershey and other end users to harmonize similar standards in order to produce a common framework of terminology and models that supports automation approaches and techniques from process to packaging.
Yep, on first blush it looks an awful lot like alphabet soup, but as the radio personality Paul Harvey so often said, “Now you know the rest of the story!” It’s not alphabet soup; it’s a carefully thought-out collection of standards and templates developed and assembled by some of the best minds in the control, automation and information disciplines with one central goal in mind: to improve your business’ visibility about what is happening right now on the plant floor.
Dave Harrold is co-founder of the AFAB Groups. He can be reached by email at [email protected].
What? You want more?
For more information on the S88/S95 connection, see these articles from past issues of Control.
“Life’s a Batch,” Greg McMillan. June, 2005, p. 80.
“Full Throttle Batch and Startup Response,” Greg McMillan. May, 2006, p. 67.
“Still Life,” Greg McMillan & Stan Weiner, PE. October, 2006, p. 103.
“Mission Possible: Control and IT Integration,” Dave Harrold. October, 2006, p. 59.
“Connecting the Enterprise,” Rich Merritt. March 2006, p. 34.
“It’s More Than Batch!,” Maurice Wilkins, PhD. Web exclusive, November 29, 2004.
“The Business Value of Automation,” Rodger Jeffrey.
Software & Integration Whitepapers. Various authors

Leaders relevant to this article: