Integrated models redefining PAT
The life sciences industry has long sought to close the loop on analytical techniques used to verify the quality of its therapies, both to automate real-time product release and optimize production throughput. But until recently, moving the necessary multivariate spectral analyses from the laboratory to the plant floor meant a massive commitment of time, resources and targeted expertise. Moreover, even after organizations committed those resources, they typically ended up with complex, fragile process analytical technology (PAT) systems requiring significant upkeep over their lifecycles.
Today, manufacturers have at their disposal a new, streamlined approach to implementing PAT by embedding the necessary chemometric models into their control systems.
“Emerson’s DeltaV Spectral PAT runs the models directly in the DeltaV control system, which allows real-time prediction of critical quality attributes,” explains Molly Firkins, DeltaV product marketing manager at Emerson. “And that means our customers can more readily monitor and perform closed-loop control on these really hard to measure but essential quality values.”
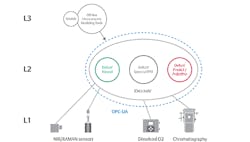
Historically, turning the 3,000 or 4,000 pieces of spectral data produced by a Raman or near infrared (NIR) probe into a univariate value usable by the control system required a layered approach. First, an engineer developed a model offline to be validated as part of the process. The model was then run on servers at Level 3 of the Purdue model to translate analyzer data into a value useful for control. Finally, that value was passed back to the controllers to adjust the process in near real-time.
Integration reduces complexity
While the layered model of traditional PAT enables closed-loop control, the connections among systems are typically quite fragile. To support such a model, operators require three or four disparate systems to communicate seamlessly. The analyzer must talk to the third-party system performing the conversion, which in turn communicates with a PAT application comparing the model and values, which itself must share data with the control system.
This configuration creates a complex web for IT and OT to implement and maintain. It also creates a wide surface area for compatibility problems. Moreover, validating all these systems is complex and time-consuming. But, with Emerson’s new DeltaV Spectral PAT, operations teams can bring spectral signals directly into the control system, where the raw spectral data is analyzed and quality assessed, and control decisions are made—all in one dramatically simplified process.
To make this all possible, an industry-leading chemometric model engine is implemented in a standard DeltaV function block running on the DeltaV Application Station. This new PAT function block is easily configured using DeltaV Control Studio, and can be easily incorporated into control strategies.
This new approach improves product quality by reducing variability—online measurements and quality calculations provide continuous data for tighter process and quality control. DeltaV Spectral PAT also increases reliability and robustness by eliminating layered solutions, multiple servers and communications dependencies of traditional approaches.
Finally, it lowers the costs and effort required to implement, maintain and validate PAT solutions. “Embedded DeltaV PAT leverages the DeltaV configuration, database and support infrastructure used by other control applications,” explains Firkins. “And process validation is simplified because applications are all part of an integrated DeltaV platform.”
Life sciences manufacturers can find themselves sitting on millions of dollars of work-in-process (WIP) inventory, while waiting on quality test results. Emerson’s DeltaV Spectral PAT technology is among the new technologies poised to unlock fully automated manufacturing and eliminate WIP altogether. The shift is happening fast, and looking toward a fully automated future will help organizations secure speed-to-market and competitive advantage in the years and decades to come.