The global energy future Part 3
My books and articles in the past concentrated on the optimization of existing processes. In order to obtain maximum energy recovery, the various solar collectors described in this series of articles all need to track the sun. The best control algorithm to use is the envelope or herding strategy, which I described in my Lessons Learned columns in the January 1998 issue ("Buildings, Bureaucrats, and Brutes,") and November 1998 issue ("Envelope Optimization") of CONTROL magazine.
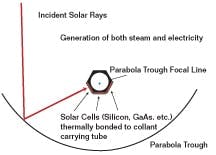
The parabolic photovoltaic (PV) collectors combine the steam generation capability of the thermal collectors with direct electricity generation by PV cells. In Figure 1 below, the silicon solar cells bonded to the coolant tube generate electricity, while the high-temperature coolant generates steam.
FIGURE 1: COMBINES SOLAR COLLECTORSolar Updraft Tower
In a number of previous articles, I have described the control systems for chimney-effect processes. (See Chapter 8.2, Vol. 2, Instrument Engineers Handbook, [IEH] 4th ed.) In high-rise buildings in the winter, this effect increases the heating load, because at the ground floor, cold air is pulled in by the chimney effect and has to be heated. I have eliminated this effect in the IBM headquarters building in New York by equalizing the inside and outside pressures at the ground floor. The same kinds of controls are applicable to solar updraft towers.
The chimney effect generates an updraft because the heavier cold ambient air enters at the bottom of the tower and pushes the lighter warm air up the chimney. This upward air flow is caused by the pressure difference between the cold air on the outside and the warm air on the inside. Static home cooling systems use this effect to pull the cold air into self-cooled homes from underground ducts.
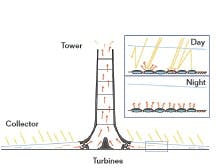
The solar updraft tower (see Figure 2 below) is an energy converter that converts solar-based thermal energy into concentrated aerodynamic energy (wind). In this system, air is heated under a circular greenhouse-like canopy. The roof of this canopy slopes upwards from the perimeter toward the center, where the tower stands. Under this canopy, the sun heats the air, which rises up the tower and generates electricity by driving an array of turbine generators.
FIGURE 2: THE SOLAR UPDRAFT TOWERThis low-tech solar energy collector concept is over a hundred years old, but the first 50 kW working model was built only in 1982.
The chimney of this model had a 10-meter (33') diameter and was 195 meters (640') tall, while the diameter of the canopy was 244 meters (800'), about 11 acres or 46,000 m2. This prototype operated for nine years and reached a maximum production of 50 kW.
Storing and Transporting Solar Energy
The storage of solar energy is an important consideration, because storage is required to compensate for the diurnal, seasonal and weather-related variations in insolation (the amount of solar energy received on a unit area). Therefore, in order to supply the continuous energy users without interruptions, the generated electricity must be stored. On small installations, hot water tanks or high-density batteries can provide storage. On mid-sized installations, pumped hydro storage can be considered. For larger installations, the compressing of air into underground caverns has been suggested. (see Chapters 8.15 & 8.41, Vol. 2 IEH, 4th ed.).
A better option is to eliminate the need for storage. This can be achieved if an electric grid is available in the area, and the utility serving the area is required to take the excess solar electricity and supplement it when more is needed. In this case, if the solar-power plant is located close to a hydroelectric or fossil power plant, it is possible to increase or decrease the fossil or hydraulic power plants rate of generation as the availability of solar energy changes.
Storing Solar Energy as Chemical Energy
A favored method of storage is to convert solar energy into chemical energy (convert it into a fuel) and store/distribute it in that form. The carriers of this chemical energy can be gases, liquids or solids.
In one process, high-temperature solar chemistry is used. Mirrors concentrate the suns rays on zinc oxide and vaporize it at a temperature of 1200 °C. The vaporized zinc is later condensed into a powder. This zinc can than be transported, and when combined with water vapor, will produce hydrogen fuel. Recombining with oxygen creates zinc oxide. A 300 kW pilot plant at the Weizmann Institute of Science (WIS) in Rehovot, Israel, has successfully produced 45 kilograms of zinc per hour, but this storage method is not yet available commercially.
Hydrogen can be generated from ammonia, from the reforming of fossil fuels or from water by electrolysis. Naturally, when made from fossil fuels, carbon dioxide is generated, which contributes to global warming, while if hydrogen is oxidized, the product is clean water, which is much needed in arid regions.
Hydrogen as a Transportation Fuel
Hydrogen is one of the means of storing solar energy in chemical form, which allows it to be used as a fuel, but before that happens, a transition period is expected, during which ethanol and other biofuels will be used. The three main American car manufacturers plan to have half their fleets run on E85 or on biodiesel fuels by 2012.
E85 is a blend of 85% ethanol and 15% gasoline. Today, out of the 170,000 gas stations in the U.S., only 2,000 have pumps for E85, and ten times that number is needed to provide most American motorists with a pump within 5 miles of their homes.
Hydrogen is stored as a liquid or as a gas compressed to some 350 to 800 atmospheres pressure (5,000 to 12,000 pounds per square inch). At atmospheric pressures, hydrogen condenses at -423 °F (-217 °C). On a weight basis, the energy content of hydrogen is 3.4 times that of gasoline. Liquid hydrogen weighs 0.59 pounds/gallon, and the energy content of one gallon of gasoline equals the heating value of 3.58 gallons of liquid hydrogen. The heating value of a kilogram of gasoline is 39,600 BTU (41.8 mJ), while the heating value of a kilogram of hydrogen is 134,616 BTU (142 mJ).
On a volume basis, hydrogen requires three times the volume of gasoline to store the same amount of energy. Hydrogen can also be stored in solids, and these reversible solid storage processes are probably the safest, but their development is still in the experimental stage. Today they are capable only of storing small amounts of energy.
Gas Stations of the Future| About the Author |